Khairy: No Medical Cannabis Products Registered Yet, MoH Welcomes Proposals For Clinical Trials
 Thirsty for JUICE content? Quench your cravings on our Instagram, TikTok and WhatsApp
Thirsty for JUICE content? Quench your cravings on our Instagram, TikTok and WhatsApp

Health Minister Khairy Jamaluddin has said that his ministry welcomes proposals from anyone possessing knowledge or scientific proof of marijuana’s medical benefits.
He also clarified that there are currently no cannabis (or marijuana) related products currently approved for use in Malaysia.
This follows on from his earlier pledge that the government would allow the drug for medical use after passing strict conditions.

“The current laws which govern cannabis and its byproducts in Malaysia – the Dangerous Drugs Act 1952, Poisons Act 1952, and Sale of Drugs Act 1952 – does not forbid cannabis use for medical purposes,” he was quoted as saying by Astro Awani.
Khairy also said that anyone with sufficient scientific evidence of the drug’s quality, safety, and efficacy can apply to the Ministry of Health affiliated National Pharmaceuticals Regulatory Agency (NPRA) and Drug Control Authority (DCA) for a license.
“This allows the product to be reviewed and then registered under the Control of Drugs and Cosmetics Regulations 1984, which then allows it to be sold in the country.”
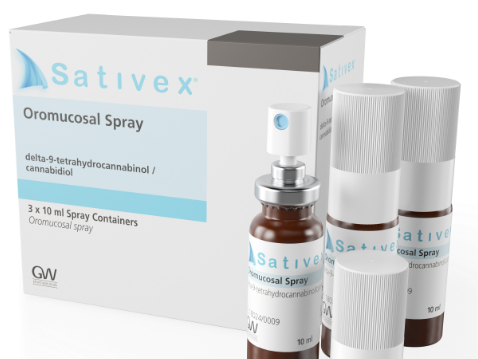
Khairy said that the ministry had registered only a single product previously – the CBD based mouth spray Sativex – back in 2014. The product is used to treat muscle spasms and spasticity linked to the incurable disease multiple sclerosis (MS).
However, Sativex was deregistered at its manufacturer GW Pharmaceuticals’ request in 2017, citing that the product was not commercially viable.


 Get Audio+
Get Audio+ Hot FM
Hot FM Kool 101
Kool 101 Eight FM
Eight FM Fly FM
Fly FM Molek FM
Molek FM

