Luggage-Size Covid-19 Breathalyser For Emergency Use Has Results Comparable To Antigen Rapid Tests
 Thirsty for JUICE content? Quench your cravings on our Instagram, TikTok and WhatsApp
Thirsty for JUICE content? Quench your cravings on our Instagram, TikTok and WhatsApp

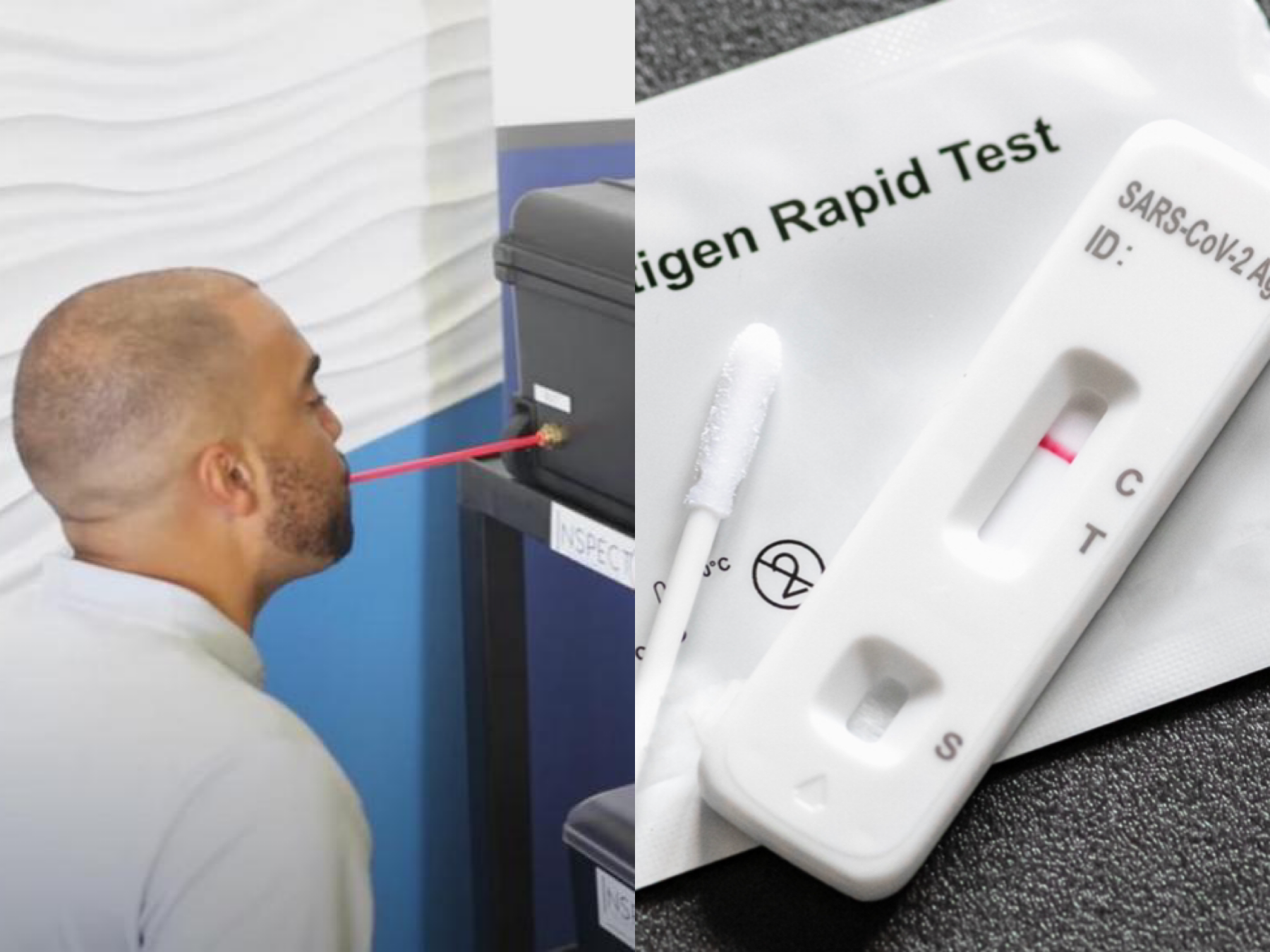
A Covid-19 breathalyser test with the ability to provide diagnostic results in three minutes has won emergency-use authorisation from the United States’ Food and Drug Administration (FDA), the agency announced on Thursday (April 14).
The device is about the size of a piece of carry-on luggage, the FDA said, and works by detecting chemical compounds in breath samples associated with Sars-CoV-2 infection.
The breathalyser’s sensitivity is comparable to that of antigen rapid tests, studies show.

The test detects chemicals associated with the virus that causes the disease in a breath sample, and if it’s positive, it should be followed up by a molecular test, the agency said in a statement.
“Today’s authorisation is yet another example of the rapid innovation occurring with diagnostic tests for Covid-19,” Dr Jeff Shuren, director of the FDA’s Centre for Devices and Radiological Health, said in the statement.
On its website, the company says the breathalyser is the first such device available for commercial use.
The company, which normally focuses on portable opioid and cannabis detection tools, expects to be able to produce about 100 of the devices each week, which can be used to evaluate about 160 samples a day.
The FDA said the test can be administered only by qualified, trained operators under the supervision of healthcare professionals with state authorisation to prescribe tests.


 Get Audio+
Get Audio+ Hot FM
Hot FM Kool 101
Kool 101 Eight FM
Eight FM Fly FM
Fly FM Molek FM
Molek FM
